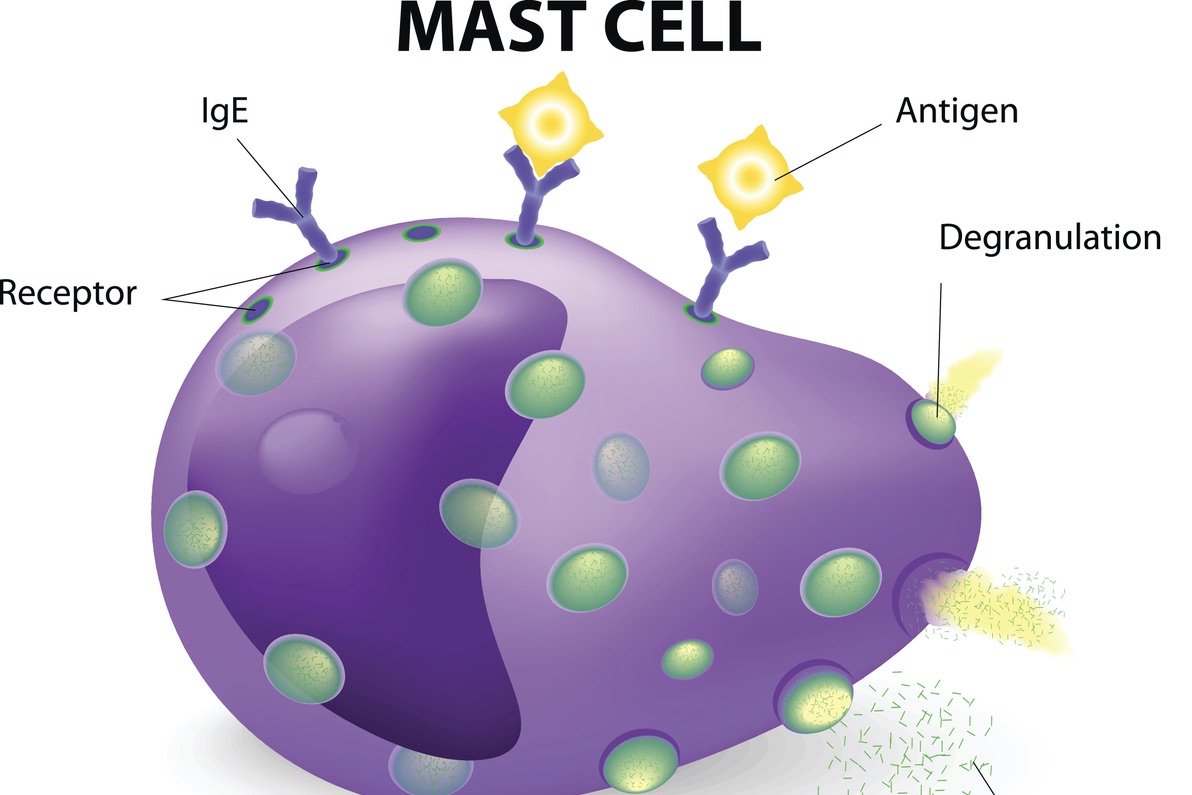
Symptoms
A Guide to Understanding Alpha-gal Syndrome’s Paradigm-Shifting Presentation
Novel Features
A Paradigm-Shifting Allergy
Alpha-gal syndrome (AGS) is a unique allergy whose paradigm-shifting features challenge past assumptions about IgE-mediated allergies. Some of these features include:
- The allergen epitope, galactose-alpha-1,3-galactose (alpha-gal), is a carbohydrate, rather than a protein (1).
- Primary sensitization to the allergen occurs through tick bites (3).
- AGS involves both food allergy and sometimes fatal hypersensitivity reactions to drugs and medical products (1,6,57).
- Onset in adulthood is more common than onset in childhood (6,57).
- Lipids (fat), which incorporate alpha-gal, are an important source of the allergen (92).
- Reactions can be delayed and typically occur 2-8 hours after the ingestion of mammalian meat (1,6,57,91).
- Reactions to other exposures, especially the infusion or injection of drugs and other medical products with mammalian ingredients, can be rapid onset or immediate (1,6,57).
Additional Atypical Features
Alpha-gal syndrome differs from other allergies in additional ways. These can include:
- Non-classical symptoms, including:
- Pronounced inter- and intra- individual variation in reactions (57)
- The profound influence of co-factors on reactivity (57)
- Anaphylactic reactions in up to 60% of cases (24,78,87,88,89)
- Reactions to airborne alpha-gal, especially fumes from cooking meat (57)
- An increase (20) or decrease (95) in reactivity based on exposure to or avoidance of additional tick bites (6,57)
- Reactions after exposure to alpha-gal in personal care and household products (6,57)
- Large, local reactions to tick or other arthropod bites (57) that often persist, sometimes for months.
- In some cases, the development of chronic spontaneous urticaria (57)
- In 3-5% of cases, the development of symptoms consistent with a mast cell syndrome (57)
- Very few (>1%) of patients report itching or swelling of the mouth or tongue (6,57,91)
*most of the southeastern U.S. and other populations with high tick exposure, including areas of the Midwest and much of the eastern U.S.
Learn More:

What Is Alpha-gal Syndrome?→

Diagnosis→
Management→
Triggers
Anything with alpha-gal in it can trigger an alpha-gal allergic reaction. The most common food triggers are mammalian meat and organs, but lard, dairy, gelatin, other mammalian byproducts and carrageenan can also cause reactions when ingested. Medical products that can trigger reactions include medications, plasma volume expanders, vaccines, and many others. Personal care and household products can also trigger reactions.
See What is Alpha-gal Found In for more information.

Mammalian meat and organs

Mammalian organs

Dairy, gelatin, and other foods derived from mammals
Drugs and other medical products

Vaccines

Personal care products
Learn More:

Mammalian Byproducts→
Carrageenan→
Classic Presentation
What are the main symptoms associated with alpha-gal syndrome and how would I know to get tested?
Based on his experience with over 2,500 patients, leading expert Scott Commins, MD, PhD has identified seven characteristics that occur in 85% of patients with alpha-gal syndrome:
(I) Onset in adult life after eating mammalian meat without problems for many years
(II) Reactions range from pruritus, localized hives or angioedema to anaphylaxis
(III) Patients can report strictly gastrointestinal symptoms (diarrhea, abdominal cramping, emesis) almost to the exclusion of cutaneous, cardiovascular, or respiratory manifestations
(IV) Reactions start 3–8 hours after eating non-primate mammalian meat (or consumption of dairy, gelatin, or other mammalian-derived products)
(V) Positive testing for alpha-gal IgE (>0.1 IU/mL)
(VI) Improvement of symptoms when adhering to an appropriate avoidance diet
(VII) Description of large local reactions to tick or other arthropod bites, often including report of an ‘index’ bite that behaved differently than prior bites (57)

Onset in adulthood

GI symptoms in isolation of other symptoms

Delayed reactions, often striking in the middle of the night
Large, itchy tick bite

Symptoms improve after a change of diet
Exceptions to this classic presentation include:
- A significant number of pediatric cases occur (91), especially in some cohorts (84). Pediatric cases may report:
- Some individuals report onset of symptoms in less than 2 hours (16% of cases in one study) (57,91)
- Not uncommonly, patients do not recall a tick bite. Approximately 50% of people who develop tick-borne diseases do not remember being bitten by a tick (57), so this isn’t particularly surprising.
- Sometimes patients recall being bitten by chiggers (57). In many cases, these “chiggers” may actually be larval tick bites, especially in the late summer and early fall.
…we have seen many children who had been diagnosed with idiopathic urticaria/anaphylaxis, or who had been specifically told that the reactions were not a result of food allergy, who had IgE antibodies to a-Gal and, in retrospect, a history consistent with delayed reactions to mammalian meat (103).

Pediatric cases: GI symptoms
Children often primarily report GI symptoms

Pediatric cases: sports
With children, there can be a strong association between reactions and sports
Symptoms
In geographic areas where tick bites are common, AGS is likely under-recognized and under-diagnosed. We suggest testing for alpha-gal IgE in tick-endemic areas as part of the evaluation for cases of idiopathic anaphylaxis, recurrent urticaria and/or angioedema, as well as recurrent, episodic gastrointestinal cramping of unestablished cause (57).
Common Symptoms
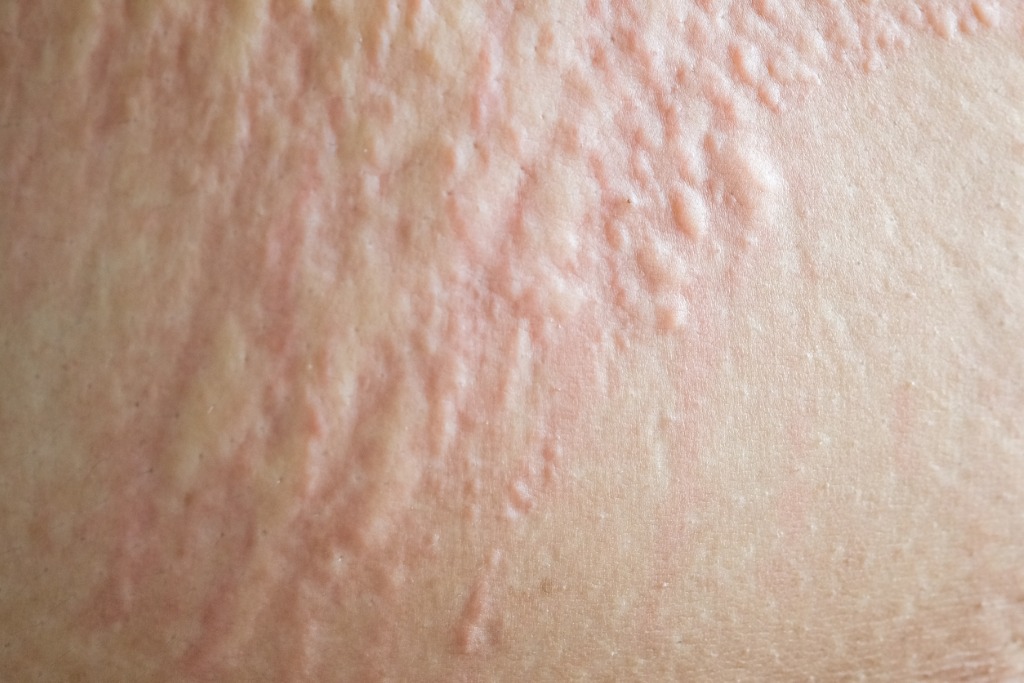
Skin
- Pruritis (itching) (57,84,90,96)
- Itching of the palms and soles of feet can be the initial symptom of a reaction (96)
- Urticaria (hives) (6,84,57,90,91,96)
- Flushing/erythema (84,90,96)
Angioedema (swelling) (6,84,90)
- Especially the palms and soles of the feet (90)
Gastrointestinal
Anaphylaxis (6,84,90,91)
- Anaphylaxis is a life-threatening condition which can involve a range of different symptoms.
- See FARE guide to anaphylaxis for information about symptoms of anaphylaxis.
Cardiovascular symptoms (90,99)
- Hypotension (low blood pressure) (90,96,110)
- Light-headedness/dizziness (90,108)
- A weak and rapid pulse
- Pre-syncope (feeling like you will faint) (90)
- Syncope (fainting, loss of consciousness) (90,97,108,110)
- Shock (98,110)
- Other cardiovascular symptoms (see Fare guide to anaphylaxis)
Respiratory symptoms (difficulty breathing) (84,90,99)
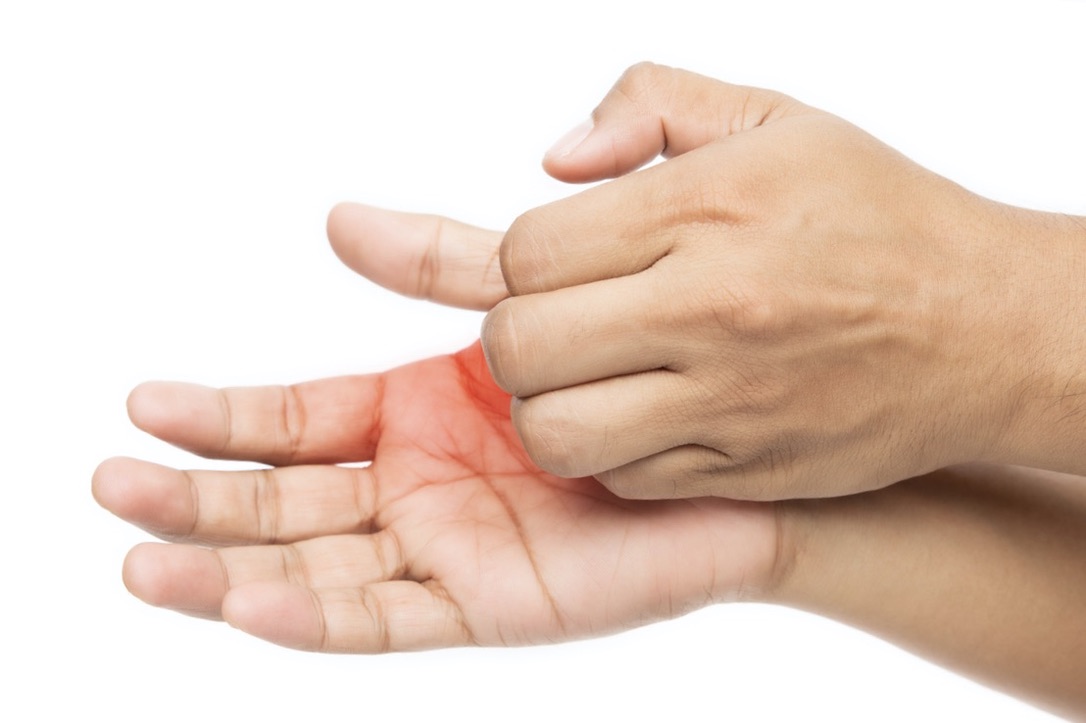
A Typical First Symptom
Itching of the palms and soles of feet, sometimes with a rash or swelling, is often the first symptom of an alpha-gal reaction (57).
According to allergist Erin McGintee, MD, who has treated over 600 patients:
About 90% of the patients present with skin symptoms. Most often it will start with the palms and the soles of the feet or the ears. They will start with intense itching, and it will progress with hives and/or flushing that can be localized or generalized. The next most common symptom that we see is actually gastrointestinal symptoms. It is very common for patients to complain of severe abdominal pain, cramping, nausea, vomiting, diarrhea–you see these in at least 60% of the patients. Cardiovascular symptoms are not uncommon. 30-40% of the patients will report feeling light-headed and I definitely have a good number of patients who lose consciousness… About 30-40% of the patients will report respiratory symptoms either involving the throat or involving the chest. (90)
During an oral food challenge involving 13 patients with AGS, Commins et al, observed:
…many of the patients with IgE to alpha-gal reported itching as their initial symptom of a food-induced allergic response. Although we were aware of this through patient reports in the past, we were not aware of the prominent palmar and plantar pruritus with erythema and often urticaria (96)
…in 3 of the subjects with IgE to alpha-gal, the initial site of itching during the meat challenge was a prior or recent tick bite site. This might suggest that basophils, mast cells, or eosinophils remain present at tick bite sites (96).
…several of the reactions showed incremental progression, in which a symptom, such as palmar erythema, might be followed in time by discrete and then systemic urticaria, which even progressed further to include gastrointestinal distress or hypotension (96).
Although all positive reactions during the meat challenge were delayed, there was variation in the time of symptom onset…patient FC-06 reported only pruritus at 292 minutes, by 6 hours he was nearly discharged before having systemic urticaria 35 minutes later. Variation was also noted in the progression of symptoms, with some patients experiencing a much more rapid course once symptoms appeared (96).
Four patients who had symptoms during the meat challenge also reported “lingering” symptoms up to 48 hours after challenge (96).
In a study of 261 adults and children reporting red meat allergy, Wilson, et al observed:
Urticaria was reported by 93%, anaphylaxis by 60%, and gastrointestinal symptoms by 64% (91).
The a-Gal syndrome is a regionally common form of food allergy that has a characteristic but not universal delay in symptom onset, includes gastrointestinal symptoms, can develop at any time in life, and is equally common in otherwise nonatopic individuals (91).
We had a cookout. I ate an average piece of steak. That night I woke up around 3:30 am in disarray. Palms blood red, itchy beyond words could describe. Half dollar hives appeared EVERYWHERE from my thighs to my belly button. Internally, it felt like a ping pong ball of fire was bouncing around my chest. Throat was tight. For an entire week after, my digestion system was beyond uncomfortable in ways I couldn’t tastefully explain. Was very ill feeling for many days.
Delayed Onset
Late-Night Anaphylaxis
“Any time you see a patient who is coming in saying they are waking up in the middle of the night with an allergic reaction, you better prove that it is not this.”
Delayed and Rapid-Onset Reactions
Unlike other allergies, alpha-gal allergic reactions are typically delayed by three-to-eight hours, at least after ingestion of red meat. The reason for this is unknown, but experts Jeffrey Wilson and Thomas Platts-Mills have proposed that the lengthy process of metabolizing the glycolipid form of alpha-gal may be involved (see below) (92).
Some reactions can be rapid or immediate. Examples include:
- In about 16% of cases, consumption of red meat can result in onset of symptoms in less than two hours (91).
- Reactions after consumption of pork kidneys (24)
- Reactions to drugs, vaccines, and other medical products, depending on how they are administered, including:
- Reactions to airborne alpha-gal, such as meat fumes, which can occur within minutes (multiple anecdotal reports).
- Reactions to topical exposures, like lotions and bandage adhesives (multiple anecdotal reports).
- Reactions to dairy products and carrageenan (multiple anecdotal reports).
Late-night Anaphylaxis
Many alpha-gal reactions occur in the middle of the night (57,90). A common pattern is for people to eat red meat for dinner, and then hours later wake-up with GI issues (most commonly severe abdominal pain), a rash or angioedema (swelling) or other symptoms of anaphylaxis (57,90). Due to the delay between eating meat and the onset of symptoms, many undiagnosed individuals fail to connect their nighttime reactions to what they ate or they attribute their reactions to food poisoning. Late-night anaphylaxis is a red flag that may suggest AGS (57,90).
The Glycolipid Hypothesis
Commins et al write:
On the basis of the process of lipid digestion, absorption, and delivery to the circulation, we believe that the 3- to 6-hour delay in symptoms after eating mammalian meat in subjects with IgE to alpha-gal is best explained by a glycolipid as the antigenic form of alpha-gal in this allergy (96).
Wilson and Platts-Mills write:
The fact that α-Gal is an oligosaccharide is often considered the most important feature of this allergen. However, it could be argued that an equally distinguishing feature of the α-Gal allergen is that it exists in the form of a glycolipid. Indeed, while there are many examples of carbohydrate allergens and of lipids modulating Th2 cell immune responses, the authors are unaware of any other common food allergen that is intrinsically part of a lipid. Thus, it is important to consider the glycolipid content of mammalian meat and organs, as well as the biochemical pathways involved in glycolipid digestion and metabolism, when considering the pathophysiology of α-Gal allergenicity (92).
Intra- and Inter- Individual Variability
Alpha-gal allergy is an any time allergy, not an every time allergy (111).
AGS is characterized by both inter-individual and intra-individual variability. Alpha-gal allergic reactions are “consistently inconsistent,” as Dr. Scott Commins puts it (6,57,101). In other words, one person’s reactions can be very different from another person’s, and also each individual can have wildly different and unpredictable reactions to the same foods.
Intra-individual variation
Not all exposures to alpha-gal result in a reaction. Some people with AGS may tolerate red meat on some occasions with few or no reactions, but have severe reactions on other occasions. This cannot be fully explained by the amount of meat eaten. (6,57,90)
Factors influencing the threshold for reactivity and severity of reactions may include:
- The quantity or form or alpha-gal in the meat (6,57,101). Organ meats (24) and fattier meats seem to be associated with more severe reactions (6,57,101).
- Recent tick bites (20,6,57,95)
- The presence or absence of co-factors (57,101)
Inter-individual variation
In addition to intra-individual variation, reactions to alpha-gal are also characterized by marked inter-individual variation.
- Many people sensitized to alpha-gal are asymptomatic (57,59)
- Others tolerate mammalian meat but have severe reactions after eating organ meats, like pork kidneys (24,59), or exposure to some medical products, like cetuximab or gelatin-based plasma volume expanders (102).
- Many cannot red meat, but tolerate dairy products and gelatin in foods (although often not in some medical products) (6,57).
- Some cannot tolerate meat, dairy, gelatin, carrageenan, or even trace amounts of alpha-gal in foods, drugs and other medical products, personal care, household, and other products (6,57).
- Titer of alpha-gal specific IgE is not strongly correlated with severity of reactions (57).
According to expert Scott Commins MD PhD, University of North Carolina:
An additional point is that some patients with the syndrome may tolerate mammalian meat on occasion with few or no symptoms but have severe reactions on others. (57)
Whether a reaction occurs to an individual exposure is inconsistent and often appears to follow no identifiable pattern for patients. The lack of consistent reactions is, in itself, almost a diagnostic hallmark. Over time, patients may experience a “progression” to more consistent reactivity and this likely reflects a new tick bite. (57)
According to experts Jörg Fischer and Mariane Hilger:
…it is assumed that the delivery route of α-gal is crucial for the risk of an allergic reaction. Especially the intravenous application of α-gal-containing drugs is associated with a high risk. Due to this potential risk, a special warning regarding the administration of α-gal- containing drugs may be needed in all individuals sensitized to α-gal, even if they are tolerant to mammalian meat and entrails (102)
The Importance of Co-Factors
Co-factors are exogenous and endogenous conditions that lower the threshold of reactivity and make allergic reactions more severe (6,23,57,101). They include:
- Exercise
- The consumption of alcohol
- The use of some medications, such as nonsteroidal anti-inflammatory drugs (NSAIDS)
- Illness
- Menses (your period)
- Stress
- Lack of sleep
Exercise and alcohol seem to be the most important co-factors for alpha-gal reactions.
According to expert Scott Commins MD PhD, University of North Carolina:
Activity, alcohol consumption, and exercise can have profound influence on reactivity. Some patients appear to have reactions that require co-factors such that they can tolerate exposures in isolation; consistent with a diagnosis of co-factor-dependent AGS (57).
A group of experts convened by the NIH write:
Patients with a-Gal food allergy report marked intraindividual variability in the dose that causes a reaction. Many describe mild or no reactions with some exposures, yet have severe symptoms on repeat exposure to the same food served in a similar amount and preparation. This variation in susceptibility, at least in part, appears to be modulated by cofactors. In keeping with many other forms of food allergy, the most prominent cofactors associated with a-Gal consumption reactions are exercise, alcohol, and nonsteroidal anti-inflammatory medications. Theoretically, exposure to these cofactors may of response, and/or contribute to the severity of the allergic responses to a-Gal (101).
Two male patients had no atopic background but food allergy to red meat and innards only, particularly at night time. The manifestation of severe symptoms was dependent on cofactors for both, such as alcohol, although mild gastrointestinal symptoms were often present during the daytime. In Patient 2, a very severe reaction occurred to goat’s lung and alcohol (105).
Anaphylaxis
Anaphylaxis is a medical emergency and requires immediate medical care!
Learn about anaphylaxis and discuss it with your healthcare provider.
For information on anaphylaxis, please click on the red buttons below.
AGS Is a Leading Cause of Anaphylaxis
- In one recent study of anaphylaxis, alpha-gal was found to be the number one trigger, accounting for 33% of cases with a definitive cause. The number two cause was all other food allergies combined at 28% (44).
- In the same study, recognition of AGS led to a reduction in the percentage of anaphylaxis cases without a definitive cause from 59% to 35% of total cases. (44)
- In a second study, nine percent of all patients referred with unexplained anaphylaxis were found to have AGS (62).
AGS is the leading cause of adult-onset allergy and anaphylaxis throughout the South and much of the eastern U.S. (12).
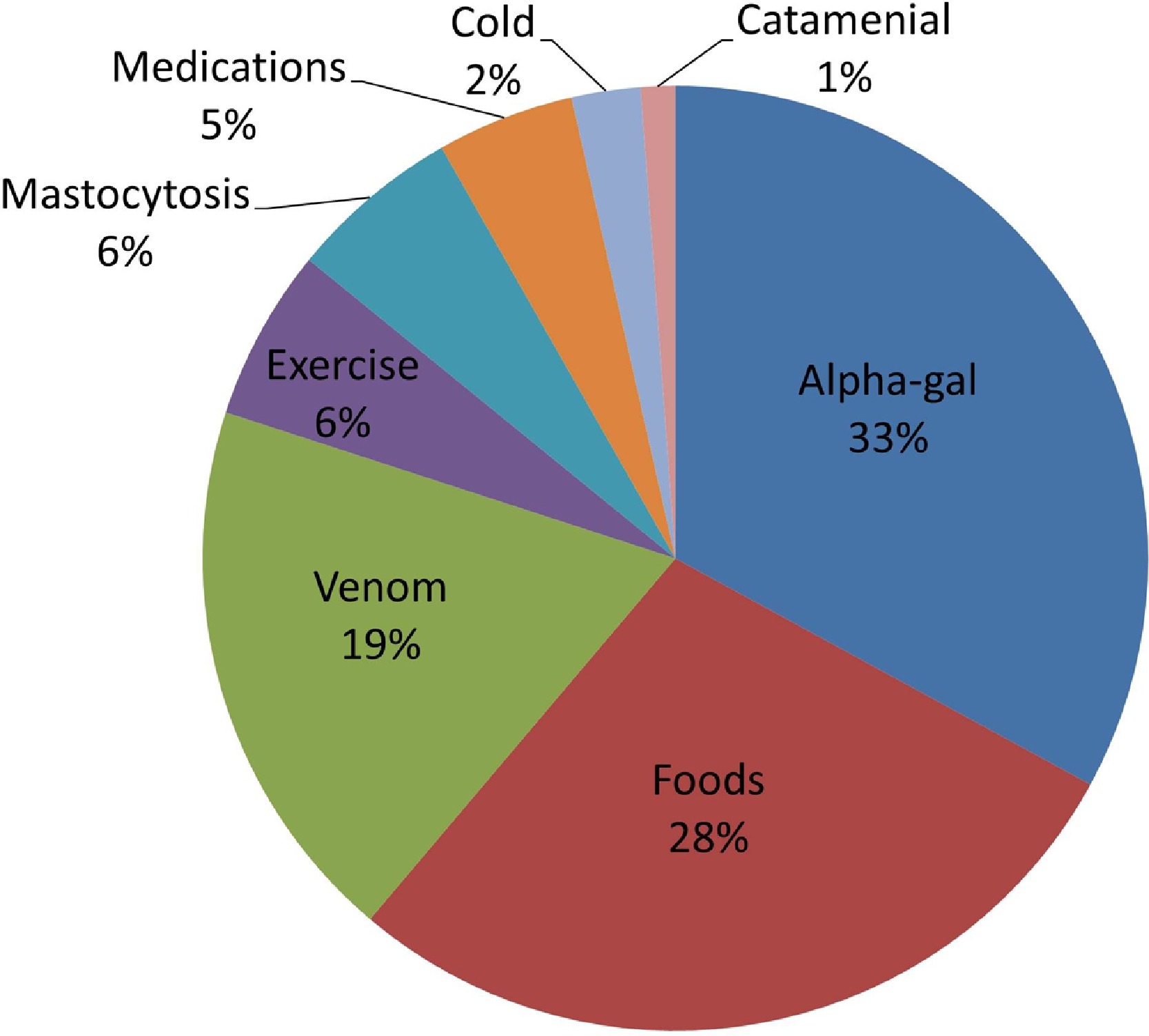
Figure 1. Etiologies of anaphylaxis based on proposed “definitive cause.” Alpha-gal, galactose-a-1,3-galactose.
Source: Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Annals of Allergy, Asthma & Immunology. 2018 Nov 1;121(5):594-7.
- About 60% of people with AGS have anaphylactic reactions (24,78,87,88,89,91), and 30-40% have cardiac and respiratory symptoms (90).
- Anaphylaxis is a serious reaction that can be fatal.
- Due to the delayed onset of alpha-gal reactions, people with AGS often experience anaphylaxis in the middle of the night, after they have gone to bed (57,90).
FARE's Guide to Anaphylaxis
Please click on the button to learn:
1. About symptoms of anaphylaxis
2. How to determine if you are having an anaphylactic reaction
3. What to do if you are experiencing anaphylaxis
Erin McGintee, MD, who has treated over 600 patients, states:
I definitely have a good percentage of patients who lose consciousness (90)
Gastrointestinal Symptoms
- Over 60% of people with AGS experience gastrointestinal symptoms (84,90,91,101), including one or more of the following:
- nausea
- diarrhea
- vomiting
- abdominal cramping
- abdominal pain
- The most common gastrointestinal symptom of AGS is cramping abdominal pain (57).
- Up to 20% of people with AGS have GI symptoms only (6,57,84).
- Some people with AGS are misdiagnosed with IBS or other gastrointestinal disorders (57).
In addition to the classic allergy symptoms, some of our patients report significant gastrointestinal distress or gynecological symptoms. These symptoms can take the form of abdominal cramping and pain, heartburn, diarrhea, nausea or vomiting and in some cases uterine cramping with spotting. It is not uncommon for a patient who has anaphylaxis to lose consciousness while moving their bowels. Some patients have reactions that are characterized almost entirely of GI or gynecological symptoms while others may not experience these types of symptoms at all.
While GI complaints are not uncommon as part of an allergic reaction, 3–20% of patients with AGS report abdominal pain, nausea, emesis, diarrhea, heartburn in isolation of cutaneous, cardiovascular, or other signs/symptoms. This pattern of signs and symptoms complicates the diagnosis as well as management of AGS because the possibility of food allergy is not obvious, but patients can be severely affected. Indeed, examples of patients with AGS who have had exploratory surgery, removal of gallbladder or appendix, and partial pancreatectomy have been reported (57).
A group of experts convened by the NIH write:
Most α-Gal meat allergy reactions reported in the literature focus on the presentation of delayed pruritus, urticaria, angioedema, and anaphylaxis. More recently, and at the workshop, researchers have described reactions that manifest with abdominal pain—both in conjunction with skin reactions and as isolated gastrointestinal reactions (101).
Anecdotally, there are patients in whom the initial symptoms were restricted to the gastrointestinal tract but over a period of weeks or months, in some cases following additional tick bites, the symptomatology expanded to include the skin and/or anaphylaxis. It is, therefore, possible that isolated abdominal pain is an underreported and underdiagnosed feature of AGS (101).
D’Souza and Howarth write:
This case highlights a novel presentation of the alpha-gal allergy, a recently observed allergy that is increasing in prevalence. It is normally difficult to diagnose given the delayed reaction and generalized symptoms (114).
With individuals with recurrent gastrointestinal symptoms it is necessary to consider alpha-gal as a potential diagnosis. It is an allergy that can be easily missed and if not diagnosed can result in anaphylaxis (114).
I react gastrointestinally only. When I have reactions, the pain starts under my ribs, comes in waves, ten times worse than labor pain, and I get horrible chills and back aches from the pain and shivering. It’s awful. My last reaction in April landed me in the hospital and they gave me pain meds instead of an epi.
Abdominal cramps for hours, threw up sometimes, and four to five days of constipation, followed by uncontrollable diarrhea. Yep, I suffered that for three years. Three years of ultrasound, X-ray and MiraLAX double dose every day. Three years of toxic hell.
My GI doctor got bit by the tick and got it too. Only then did she call me in for the test.
It was always the upper abdomen, to the right side. It felt like a constant burning. It would last for a couple of hours, and there was nothing I could do to alleviate the pain. I had diarrhea every day. I was tested for gallbladder issues, pancreatitis, leukemia, everything except alpha-gal syndrome. I and was eventually diagnosed w IBS. I lost 25 lbs over a period of 6 months and didn’t need to. It took 10 yrs for an accurate diagnosis.
I have been symptom-free for 2 years since I eliminated all mammal meat, dairy, and anything else that might have a slight hint of any kind of mammal byproduct.
Reactions to Airborne Alpha-gal
There is very little published information on reactions to airborne alpha-gal. However, many people with AGS report them. Often, they describe them as almost immediate and severe. Some describe losing consciousness.
- Although no data on the prevalence of reactions to airborne alpha-gal have been published, informally some experts state that anywhere from 10-30% of their patients report them (111, 115).
- The most commonly reported trigger is fumes from cooking meat (57), especially from grills, barbeques, and sometimes frying meat, but people with AGS report that many other forms of airborne alpha-gal can also trigger reactions (multiple anecdotal reports).
Expert Scott Commins, MD, PhD writes:
Patients do report symptoms with exposure to fumes from mammalian meats/fats being cooked; however, no blinded challenges have been published to definitively document the airborne (droplet) route of exposure. Interestingly, experience suggests fumes may pose a more potent risk to reactive patients than moderate levels of pet dander exposure (57).
The scariest part of this whole thing for me has been the fume (aka airborne reactions).
Learn More:

Read Patient Accounts of Airborne Reactions to Alpha-gal→
Arthritis/Joint Pain
In areas where Lone Star ticks are present, and in patients with risk factors for tick exposure, alpha gal IgE reactivity should be considered and tested for as part of a “tick panel” in patients who present with symptoms of potential rheumatologic diseases (60).
Arthritis and other forms of joint pain seem to be under-recognized manifestations of alpha-gal syndrome (6,57,60). Very little has been published on it, but many individuals with AGS report that their arthritis improves or resolves in response to an avoidance diet.
- One study of patients referred to a rheumatology practice with known tick exposure or risk for exposure to ticks, found that over 140 patients tested positive for alpha-gal IgE (60).
- Of the 38 patients seen in follow-up:
Chronic Urticaria
Though larger studies are needed, we postulate a benefit for screening patients undergoing evaluation for chronic urticaria who have a suggestive dietary history and live in regions where the α-gal syndrome is common (104).
Diagnosis by skin testing is difficult, but alpha-gal should be considered in children with recurrent idiopathic urticaria living in the southeastern United States. (112).
Alpha-gal syndrome can be easily misdiagnosed as chronic urticaria (104,112).
- In one study, a majority of patients who had been diagnosed with chronic urticaria or chronic idiopathic urticaria prior to being diagnosed with AGS experienced complete or partial improvement with an avoidance diet (104).
- The conclusions of this study are supported by an earlier case study (107).
- A third study found that of 83 patients presenting with chronic urticaria at an urban, research center in Germany, only three were sensitized to alpha-gal and none had clinical AGS (106).
Some patients with AGS go on to develop chronic spontaneous urticaria despite an avoidance diet (57).
In areas where AGS is common, there may be a benefit to screening patients with chronic urticaria for alpha-gal IgE (104,112)
Expert Scott Commins, MD, PhD writes:
Distinguishing AGS from chronic spontaneous urticaria (CSU) can be challenging, leading to misdiagnosis and, in some cases, the two entities may overlap. Analysis of other cohorts have found that AGS is not a cause of unrecognized CSU. In keeping with both possibilities, we have seen patients develop AGS and achieve control of reactions through an appropriate avoidance diet yet within weeks or months they develop CSU (despite the continued mammalian avoidance diet) (57).
References
1. Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, Woodfolk JA, Platts-Mills TA. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1, 3-galactose. Journal of Allergy and Clinical Immunology. 2009 Feb 1;123(2):426-33.
2. Commins S, Lucas S, Hosen J, Satinover SM, Borish L, Platts-Mills TA. Anaphylaxis and IgE antibodies to galactose-alpha-1, 3-galactose (alphaGal): insight from the identification of novel IgE ab to carbohydrates on mammalian proteins. Journal of Allergy and Clinical Immunology. 2008 Feb 1;121(2):S25.
3. Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1, 3-galactose. Journal of Allergy and Clinical Immunology. 2011 May 1;127(5):1286-93.
4. Soh JY, Huang CH, Lee BW. Carbohydrates as food allergens. Asia Pacific Allergy. 2015 Jan 1;5(1):17-24.
5. Levin M, Apostolovic D, Biedermann T, Commins SP, Iweala OI, Platts-Mills TA, Savi E, van Hage M, Wilson JM. Galactose α-1, 3-galactose phenotypes: Lessons from various patient populations. Annals of Allergy, Asthma & Immunology. 2019 Jun 1;122(6):598-602.
6. Platts-Mills TA, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and management of patients with the α-Gal syndrome. The Journal of Allergy and Clinical Immunology: In Practice. 2020 Jan 1;8(1):15-23.
7. Commins SP. Invited commentary: alpha-gal allergy: tip of the iceberg to a pivotal immune response. Current allergy and asthma reports. 2016 Sep 1;16(9):61.
8. Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, Karim S. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Frontiers in immunology. 2019 May 17;10:1056.
9. Monzón JD, Atkinson EG, Henn BM, Benach JL. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome biology and evolution. 2016 May 1;8(5):1351-60.
10. Raghavan RK, Peterson AT, Cobos ME, Ganta R, Foley D. Current and future distribution of the lone star tick, Amblyomma americanum (L.)(Acari: Ixodidae) in North America. PLoS One. 2019 Jan 2;14(1):e0209082.
11. Wilson JM, Platts-Mills TA. Red meat allergy in children and adults. Current opinion in allergy and clinical immunology. 2019 Jun 1;19(3):229-35.
12. Commins, SP. (2018). Retrieved from: More people developing red meat allergy from tick bites. CBS News
13. Flaherty MG, Threats M, Kaplan SJ. Patients’ Health Information Practices and Perceptions of Provider Knowledge in the Case of the Newly Discovered Alpha-gal Food Allergy. Journal of Patient Experience. 2020 Feb;7(1):132-9.
14. Flaherty MG, Kaplan SJ, Jerath MR. Diagnosis of life-threatening alpha-gal food allergy appears to be patient driven. Journal of primary care & community health. 2017 Oct;8(4):345-8.
15. Cabezas-Cruz A, Hodžić A, Román-Carrasco P, Mateos-Hernández L, Duscher GG, Sinha DK, Hemmer W, Swoboda I, Estrada-Peña A, De La Fuente J. Environmental and molecular drivers of the α-Gal syndrome. Frontiers in Immunology. 2019 May 31;10:1210.
16. Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Current opinion in allergy and clinical immunology. 2013 Aug;13(4):354.
17. Stoltz LP, Cristiano LM, Dowling AP, Wilson JM, Platts-Mills TA, Traister RS. Could chiggers be contributing to the prevalence of galactose-alpha-1, 3-galactose sensitization and mammalian meat allergy?. The journal of allergy and clinical immunology. In practice. 2019 Feb;7(2):664.
18. Arkestål K, Sibanda E, Thors C, Troye-Blomberg M, Mduluza T, Valenta R, Grönlund H, van Hage M. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-α1, 3-galactose. Journal of Allergy and Clinical Immunology. 2011 Apr 1;127(4):1024-8.
19. Chinuki Y, Ishiwata K, Yamaji K, Takahashi H, Morita E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy. 2016 Mar;71(3):421-5.
20. Hashizume H, Fujiyama T, Umayahara T, Kageyama R, Walls AF, Satoh T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-α-1, 3-galactose carbohydrate IgE antibody levels: a retrospective cohort study in a single institution. Journal of the American Academy of Dermatology. 2018 Jun 1;78(6):1135-41.
21. Bianchi, John (2019). Personal communication
22. Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, Cordebar V, Morel‐Codreanu F, Petit N, Moneret‐Vautrin DA, Kanny G. Anaphylaxis to pork kidney is related to I g E antibodies specific for galactose‐alpha‐1, 3‐galactose. Allergy. 2012 May;67(5):699-704.
23. Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1, 3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. Journal of allergy and clinical immunology. 2014 Sep 1;134(3):755-9.
24. Fischer J, Yazdi AS, Biedermann T. Clinical spectrum of α-Gal syndrome: from immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergo journal international. 2016 Mar 1;25(2):55-62.
25. McPherson TB, Liang H, Record RD, Badylak SF. Galα (1, 3) Gal epitope in porcine small intestinal submucosa. Tissue engineering. 2000 Jun 1;6(3):233-9.
26. Fujiwara M, Araki T. Immediate anaphylaxis due to beef intestine following tick bites. Allergology International. 2019;68(1):127-9.
27. Caponetto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-α-1, 3-galactose. The Journal of Allergy and Clinical Immunology: In Practice. 2013 May 1;1(3):302-3.
28. Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1, 3-galactose. Journal of Allergy and Clinical Immunology. 2012 May 1;129(5):1334-42.
29. Kaman K, Robertson D. ALPHA-GAL ALLERGY; MORE THAN MEAT?. Annals of Allergy, Asthma & Immunology. 2018 Nov 1;121(5):S115.
30. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1, 3-galactose. New England journal of medicine. 2008 Mar 13;358(11):1109-17.
31. Berg EA, Platts-Mills TA, Commins SP. Drug allergens and food—the cetuximab and galactose-α-1, 3-galactose story. Annals of Allergy, Asthma & Immunology. 2014 Feb 1;112(2):97-101.
32. Dunkman WJ, Rycek W, Manning MW. What does a red meat allergy have to do with anesthesia? Perioperative management of alpha-gal syndrome. Anesthesia & Analgesia. 2019 Nov 1;129(5):1242-8.
33. Pfützner W, Brockow K. Perioperative drug reactions–practical recommendations for allergy testing and patient management. Allergo journal international. 2018 Jun 1;27(4):126-9.
34. Dewachter P, Kopac P, Laguna JJ, Mertes PM, Sabato V, Volcheck GW, Cooke PJ. Anaesthetic management of patients with pre-existing allergic conditions: a narrative review. British journal of anaesthesia. 2019 Jul 1;123(1):e65-81.
35. Popescu FD, Cristea OM, IONICĂ FE, Vieru M. DRUG ALLERGIES DUE TO IgE SENSITIZATION TO α-GAL. magnesium. 2018;2017:47-8.
36. Swiontek K, Morisset M, Codreanu-Morel F, Fischer J, Mehlich J, Darsow U, Petitpain N, Biedermann T, Ollert M, Eberlein B, Hilger C. Drugs of porcine origin—A risk for patients with α-gal syndrome?. The Journal of Allergy and Clinical Immunology: In Practice. 2019 May 1;7(5):1687-90.
37. Vidal C, Mendez-Brea P, Lopez-Freire S, Gonzalez-Vidal T. Vaginal Capsules: An Unsuspected Probable Source of Exposure to α-Gal. Journal of investigational allergology & clinical immunology. 2016;26(6):388.
38. Muglia C, Kar I, Gong M, Hermes-DeSantis ER, Monteleone C. Anaphylaxis to medications containing meat byproducts in an alpha-gal sensitized individual. The journal of allergy and clinical immunology. In practice. 2015;3(5):796.
39. Akella K, Patel H, Wai J, Roppelt H, Capone D. Alpha Gal-Induced Anaphylaxis to Herpes Zoster Vaccination. Chest. 2017 Oct 1;152(4):A6.
40. Bakhtiar MF, Leong KW, Kwok FY, Hui MT, Tang MM, Joseph CT, Bathumana‐Appan PP, Nagum AR, ZHM Y, Murad S. P66: ALLERGIC REACTION TO BOVINE GELATIN COLLOID: THE ROLE OF IMMUNOGLOBULIN E TOWARDS GALACTOSE‐ALPHA‐1, 3‐GALACTOSE: IMPLICATIONS BEYOND RED MEAT ALLERGIES. Internal Medicine Journal. 2017 Sep;47:24-.
41. Bradfisch F, Pietsch M, Forchhammer S, Strobl S, Stege HM, Pietsch R, Carstens S, Schäkel K, Yazdi A, Saloga J. Case series of anaphylactic reactions after rabies vaccinations with gelatin sensitization. Allergo Journal International. 2019 Jun 1;28(4):103-6.
42. Stone CA, Commins SP, Choudhary S, Vethody C, Heavrin JL, Wingerter J, Hemler JA, Babe K, Phillips EJ, Norton AE. Anaphylaxis after vaccination in a pediatric patient: further implicating alpha-gal allergy. The Journal of Allergy and Clinical Immunology: In Practice. 2019 Jan 1;7(1):322-4.
43. Stone CA, Hemler JA, Commins SP, Schuyler AJ, Phillips EJ, Peebles RS, Fahrenholz JM. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism. Journal of Allergy and Clinical Immunology. 2017 May 1;139(5):1710-3.
44. Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Annals of Allergy, Asthma & Immunology. 2018 Nov 1;121(5):594-7.
45. Ankersmit HJ, Copic D, Simader E. When meat allergy meets cardiac surgery: A driver for humanized bioprosthesis. The Journal of thoracic and cardiovascular surgery. 2017 Oct 1;154(4):1326-7.
46. Hawkins RB, Frischtak HL, Kron IL, Ghanta RK. Premature bioprosthetic aortic valve degeneration associated with allergy to galactose‐alpha‐1, 3‐galactose. Journal of cardiac surgery. 2016 Jul;31(7):446-8.
47. Kleiman AM, Littlewood KE, Groves DS. Delayed anaphylaxis to mammalian meat following tick exposure and its impact on anesthetic management for cardiac surgery: a case report. A&A Practice. 2017 Apr 1;8(7):175-7.
48. Mozzicato SM, Tripathi A, Posthumus JB, Platts-Mills TA, Commins SP. Porcine or bovine valve replacement in three patients with IgE antibodies to the mammalian oligosaccharide galactose-alpha-1, 3-galactose. The journal of allergy and clinical immunology. In practice. 2014 Sep;2(5):637.
49. Mangold A, Szerafin T, Hoetzenecker K, Hacker S, Lichtenauer M, Niederpold T, Nickl S, Dworschak M, Blumer R, Auer J, Ankersmit HJ. Alpha-Gal specific IgG immune response after implantation of bioprostheses. The Thoracic and cardiovascular surgeon. 2009 Jun;57(04):191-5.
50. Fischer J, Eberlein B, Hilger C, Eyer F, Eyerich S, Ollert M, Biedermann T. Alpha‐gal is a possible target of IgE‐mediated reactivity to antivenom. Allergy. 2017 May;72(5):764-71.
51. Rizer J, Brill K, Charlton N, King J. Acute hypersensitivity reaction to Crotalidae polyvalent immune Fab (CroFab) as initial presentation of galactose-α-1, 3-galactose (α-gal) allergy. Clinical Toxicology. 2017 Aug 9;55(7):668-9.
52. Farooque S, Kenny M, Marshall SD. Anaphylaxis to intravenous gelatin‐based solutions: a case series examining clinical features and severity. Anaesthesia. 2019 Feb;74(2):174-9.
53. Lied GA, Lund KB, Storaas T. Intraoperative anaphylaxis to gelatin-based hemostatic agents: a case report. Journal of asthma and allergy. 2019;12:163.
54. Tobacman JK. The common food additive carrageenan and the alpha-gal epitope. Journal of Allergy and Clinical Immunology. 2015 Dec 1;136(6):1708-9.
55. Tarlo SM, Dolovich J, Listgarten C. Anaphylaxis to carrageenan: A pseudo–latex allergy. Journal of allergy and clinical immunology. 1995 May 1;95(5):933-6.
56. Steinke JW, Platts-Mills TAE, Schuyler A, Commins SP. Reply to “The common food additive carrageenan and the alpha-gal epitope”. Journal of Allergy and Clinical Immunology. 2015 Oct 28;136(6):1709-10
57. Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Review of Clinical Immunology. 2020 Jul 9:1-1.
58. Galili, U., & Avila, J. L. (Eds.). (2012). α–Gal and Anti–Gal: α1, 3–Galactosyltransferase, α–Gal Epitopes, and the Natural Anti–Gal Antibody Subcellular Biochemistry (Vol. 32). Springer Science & Business Media.
59. Fischer J, Lupberger E, Hebsaker J, Blumenstock G, Aichinger E, Yazdi AS, Reick D, Oehme R, Biedermann T. Prevalence of type I sensitization to alpha‐gal in forest service employees and hunters. Allergy. 2017 Oct;72(10):1540-7.
60. SAT0456 SERO-REACTIVITY TO GALACTOSE-ALPHA-1,3-GALACTOSE AND CLINICAL PRESENTATIONS OF PATIENTS SEEN IN A RHEUMATOLOGY OUTPATIENT PRACTICE. Annals of the Rheumatic Diseases 2019 Jun 15;78:1317-8.
61. Bianchi J, Walters A, Fitch ZW, Turek JW. Alpha-gal syndrome: Implications for cardiovascular disease. Global Cardiology Science and Practice. 2020 Feb 9;2019(3).
62. Carter MC, Ruiz‐Esteves KN, Workman L, Lieberman P, Platts‐Mills TA, Metcalfe DD. Identification of alpha‐gal sensitivity in patients with a diagnosis of idiopathic anaphylaxis. Allergy. 2018 May;73(5):1131-4.
63. Commins, SP retrieved from: https://twitter.com/scott_commins/status/1232777851541360643
64. I-TICK Surveillance. Retrieved from: https://twitter.com/ITickUIUC/status/1282799807996854278
65. Wickner PG, Commins SP. The first 4 Central American cases of delayed meat allergy with galactose-alpha-1, 3-galactose positivity clustered among field biologists in Panama. Journal of Allergy and Clinical Immunology. 2014 Feb 1;133(2):AB212.
66. Ohshita N, Ichimaru Y, Gamoh S, Tsuji K, Kishimoto N, Tsutsumi YM, Momota Y. Management of infusion reactions associated with cetuximab treatment: A case report. Molecular and Clinical Oncology. 2017 Jun 1;6(6):853-5.
67. Stein D, Schuyler A, Commins S, Behm B, Chitnavis M. P-002 YI First Dose IgE-Mediated Allergy to Infliximab Due to Galactose-α-1, 3-Galactose Allergy. Inflammatory Bowel Diseases. 2016 Mar 1;22:S9-10.
68. Van Tine BA, Govindarajan R, Attia S, Somaiah N, Barker SS, Shahir A, Barrett E, Lee P, Wacheck V, Ramage SC, Tap WD. Incidence and management of olaratumab infusion-related reactions. Journal of oncology practice. 2019 Nov;15(11):e925-33.
69. Venturini M, Lobera T, Sebastián A, Portillo A, Oteo JA. IgE to α-Gal in Foresters and Forest Workers From La Rioja, North of Spain. Journal of investigational allergology & clinical immunology. 2018;28(2):106.
70. Apostolovic D, Tran TA, Hamsten C, Starkhammar M, Cirkovic Velickovic T, van Hage M. Immunoproteomics of processed beef proteins reveal novel galactose‐α‐1, 3‐galactose‐containing allergens. Allergy. 2014 Oct;69(10):1308-15.
71. Khora SS, Navya P. Bioactive Polysaccharides from Marine Macroalgae. Encyclopedia of Marine Biotechnology. 2020 Aug 4.
72. Gowda DC, Glushka J, Halbeek HV, Thotakura RN, Bredehorst R, Vogel CW. N-linked oligosaccharides of cobra venom factor contain novel α (1-3) galactosylated Lex structures. Glycobiology. 2001 Mar 1;11(3):195-208.
73. Hodžić A, Mateos-Hernández L, Fréalle E, Román-Carrasco P, Alberdi P, Pichavant M, Risco-Castillo V, Le Roux D, Vicogne J, Hemmer W, Auer H. Infection with Toxocara canis Inhibits the Production of IgE Antibodies to α-Gal in Humans: Towards a Conceptual Framework of the Hygiene Hypothesis?. Vaccines. 2020 Jun;8(2):167.
74. Taguchi T, Kitajima K, Muto Y, Inoue S, Khoo KH, Morris HR, Dell A, Wallace RA, Selman K, Inoue Y. A precise structural analysis of a fertilization-associated carbohydrate-rich glycopeptide isolated from the fertilized eggs of euryhaline killi fish (Fundulus heteroclitus). Novel penta-antennary N-glycan chains with a bisecting N-acetylglucosaminyl residue. Glycobiology. 1995 Sep 1;5(6):611-24.
75. Shao Y, Yu Y, Pei CG, Qu Y, Gao GP, Yang JL, Zhou Q, Yang L, Liu QP. The expression and distribution of α-Gal gene in various species ocular surface tissue. International journal of ophthalmology. 2012;5(5):543.
76. Chauhan PS, Saxena A. Bacterial carrageenases: an overview of production and biotechnological applications. 3 Biotech. 2016 Dec 1;6(2):146.
77. USDA Carrageenan Handling/Processing
78. van Nunen S. Galactose-alpha-1, 3-galactose, mammalian meat and anaphylaxis: a world-wide phenomenon?. Current Treatment Options in Allergy. 2014 Sep 1;1(3):262-77.
79. Wilson JM, Nguyen AT, Schuyler AJ, Commins SP, Taylor AM, Platts-Mills TA, McNamara CA. IgE to the mammalian oligosaccharide galactose-α-1, 3-galactose is associated with increased atheroma volume and plaques with unstable characteristics—brief report. Arteriosclerosis, thrombosis, and vascular biology. 2018 Jul;38(7):1665-9.
80. Wilson JM, McNamara CA, Platts-Mills TA. IgE, α-Gal and atherosclerosis. Aging (Albany NY). 2019 Apr 15;11(7):1900.
81. Tina Merritt, MD, personal communication.
82. Hodgeman N, Horn CL, Paredes A. An Unusual Mimicker of Irritable Bowel Disease: 1855. American Journal of Gastroenterology. 2019 Oct 1;114(2019 ACG Annual Meeting Abstracts):S1039.
83. Bensinger A, Green P. Mammalian Meat Allergy Masquerading as IBS-D: 1846. American Journal of Gastroenterology. 2019 Oct 1;114(2019 ACG Annual Meeting Abstracts):S1036-7.
84. Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha‐gal IgE levels and alpha‐gal IgE: Total IgE ratio and oral food challenge‐proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatric Allergy and Immunology. 2018 Dec;29(8):841-9.
85. Armstrong P, Binder A, Amelio C, Kersh G, Biggerstaff B, Beard C, Petersen L, Commins S. Descriptive Epidemiology of Patients Diagnosed with Alpha-gal Allergy—2010–2019. Journal of Allergy and Clinical Immunology. 2020 Feb 1;145(2):AB145.
86. Pointreau Y, Commins SP, Calais G, Watier H, Platts-Mills TA. Fatal infusion reactions to cetuximab: role of immunoglobulin E–mediated anaphylaxis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012 Jan 20;30(3):334.
87. van Nunen S. Tick-induced allergies: mammalian meat allergy, tick anaphylaxis and their significance. Asia Pacific Allergy. 2015 Jan 1;5(1):3-16.
88. van Nunen S, O’Connor K, Fernando S, Clarke L, Boyle R. THE ASSOCIATION BETWEEN IXODES HOLOCYCLUS TICK BITE REACTIONS AND RED MEAT ALLERGY: P17. Internal Medicine Journal. 2007 Nov 1;37.
89. Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. The Medical journal of Australia. 2009 May 4;190(9):510-1.
90. Meat Allergy Tirggered by a Tick Bite with Eri McGintee retrieved from: https://youtu.be/hj96Vvr1WhQ
91. Wilson JM, Schuyler AJ, Workman L, Gupta M, James HR, Posthumus J, McGowan EC, Commins SP, Platts-Mills TA. Investigation into the α-gal syndrome: characteristics of 261 children and adults reporting red meat allergy. The Journal of Allergy and Clinical Immunology: In Practice. 2019 Sep 1;7(7):2348-58.
92. Wilson JM, Platts-Mills TA. The oligosaccharide galactose-α-1, 3-galactose and the α-Gal syndrome: insights from an epitope that is causal in immunoglobulin E-mediated immediate and delayed anaphylaxis. Eur Med J. 2018;3:89-98.
93. O’Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. Journal of Clinical Oncology. 2007 Aug 20;25(24):3644-8.
94. Dupont B, Mariotte D, Moldovan C, Grellard JM, Vergnaud MC, Laroche D, Gervais R. Case report about fatal or near-fatal hypersensitivity reactions to cetuximab: anticetuximab IgE as a valuable screening test. Clinical Medicine Insights: Oncology. 2014 Jan;8:CMO-S13897.
95. Kim MS, Straesser MD, Keshavarz B, Workman L, McGowan EC, Platts-Mills TA, Wilson JM. IgE to galactose-α-1, 3-galactose wanes over time in patients who avoid tick bites. The Journal of Allergy and Clinical Immunology: In Practice. 2020 Jan 1;8(1):364-7.
96. Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, Mozzicato S, Platts-Mills TA. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1, 3-galactose. Journal of allergy and clinical immunology. 2014 Jul 1;134(1):108-15.
97. Abreu C, Bartolomé B, Cunha L, Falcao H. Galactose-α-1, 3-galactose allergy: a rare syndrome and an atypical presentation. European annals of allergy and clinical immunology. 2018 Jul;50(4):190-2.
98. Backer E, Carroll J. A Case of Unexplained Shock Following Steak Consumption. Chest. 2016 Oct 1;150(4):1139A.
99. Kiewiet MG, Apostolovic D, Starkhammar M, Grundström J, Hamsten C, van Hage M. Clinical and serological characterization of the α-Gal syndrome-importance of atopy for symptom severity in a European cohort. The Journal of Allergy and Clinical Immunology: In Practice. 2020 Mar 3.
100. Schimmel J, Fawaz B, Renzi M, Heymann WR, van Nunen S, Manders S. Mammalian meat allergy-beyond urticaria?. International journal of dermatology. 2020 May 7.
101. Platts-Mills TA, Commins SP, Biedermann T, van Hage M, Levin M, Beck LA, Diuk-Wasser M, Jappe U, Apostolovic D, Minnicozzi M, Plaut M. On the cause and consequences of IgE to galactose-α-1, 3-galactose: a Report from the National Institute of Allergy and Infectious Disease Workshop on Understanding IgE-Mediated Mammalian Meat Allergy. Journal of Allergy and Clinical Immunology. 2020 Feb 10.
102. Fischer J, Hilger C. Alpha-gal syndrome: clinical presentation, new concepts, and unmet needs. Current Treatment Options in Allergy. 2017 Sep 1;4(3):303-11.
103. Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, Tripathi A, Lane CJ, Matos L, Heymann PW, Commins SP. Galactose-α-1, 3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013 May 1;131(5):e1545-52.
104. Pollack K, Zlotoff BJ, Borish LC, Commins SP, Platts-Mills TA, Wilson JM. α-Gal syndrome vs chronic urticaria. JAMA dermatology. 2019 Jan 1;155(1):115-6.
105. Bircher AJ, Hofmeier KS, Link S, Heijnen I. Food allergy to the carbohydrate galactose-alpha-1, 3-galactose (alpha-gal): four case reports and a review. European Journal of Dermatology. 2017 Jan 1;27(1):3-9.
106. Maurer M, Church MK, Metz M, Starkhammar M, Hamsten C, van Hage M. Galactose-α-1, 3-Galactose Allergy Is Not a Hitherto Unrecognized Cause of Chronic Spontaneous Urticaria. International archives of allergy and immunology. 2015;167(4):250-2.
107. Ghahramani GK, Temprano J. Tick bite‐related meat allergy as a cause of chronic urticaria, angioedema, and anaphylaxis in endemic areas. International Journal of Dermatology. 2015 Jan 23;2(54):e64-5.
108. Tankersley M, DeJarnatt A, DeJarnatt R. Alpha-gal hypersensitivity: a case series from good ol’Rocky Top Tennessee. Journal of Allergy and Clinical Immunology. 2016 Feb 1;137(2):AB55.
109. Backer E, Carroll J. 1742: DELAYED ANAPHYLAXIS FOLLOWING MAMMALIAN MEAT CONSUMPTION AN EVOLVING VECTOR-BORN PROCESS. Critical Care Medicine. 2016 Dec 1;44(12):511.
110. Hilger C, Fischer J, Wölbing F, Biedermann T. Role and mechanism of galactose-alpha-1, 3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Current allergy and asthma reports. 2019 Jan 1;19(1):3.
111. van Nunen, S. Mammalian Meat Allergy after Tick Bite and Tick Anaphylaxis in Australia. International Tick Symposium (2018). 2018. Retrieved from https://www.youtube.com/watch?v=C4fzZO9UlzE&list=LLcoL9fUr713IGKBwo-CELVw&index=21 @1:09:00
112. James H, Kennedy JL, Platts-Mills T, Stallings AP, Workman LJ, Tripathi A, Pochan S, Lane C, Matos LA, Eapen SS, McBride DC. Pediatric Alpha-Gal: IgE Antibodies to Galactose-Alpha-1, 3-Galactose in Children Presenting with Delayed Urticaria or Anaphylaxis. Journal of Allergy and Clinical Immunology. 2013 Feb 1;131(2):AB217.
113. Patel J, Wilson J. M310 ALPHA GAL SYNDROME MANIFESTING AS ISOLATED GASTROINTESTINAL SYMPTOMS IN A MARRIED COUPLE. Annals of Allergy, Asthma & Immunology. 2020 Nov 1;125(5):S99-100.
114. D’Souza M, Lania-Howarth M. M306 RECURRING GASTROINTESTINAL SYMPTOMS AS A NOVEL PRESENTATION OF ALPHA-GAL ALLERGY. Annals of Allergy, Asthma & Immunology. 2020 Nov 1;125(5):S98-9.
115. Scott Commins, MD, PhD, personal communication
All the information on alphagalinformation.org is provided in good faith, but we, the creators and authors of the Alpha-gal Information website offer no representation or warranty, explicit or implied, of the accuracy, adequacy, validity, reliability, availability, or completeness of any information on this site. Under no circumstances should we have any liability for any loss or damage incurred by you as a result of relying on information provided here. We are not physicians or medical professionals, researchers, or experts of any kind. Information provided in this website may contain errors and should be confirmed by a physician. Information provided here is not medical advice. It should not be relied upon for decisions about diagnosis, treatment, diet, food choice, nutrition, or any other health or medical decisions. For advice about health or medical decisions including, but not limited to, diagnosis, treatment, diet, and health care consult a physician.
READ FULL DISCLAIMER>